Vaccine Breakthrough A Strain-Agnostic Solution Revolutionizing Global Health
Vaccine Breakthrough A Strain-Agnostic Solution Revolutionizing Global Health - Strain-Agnostic Vaccines: Unlocking Global Health Potential
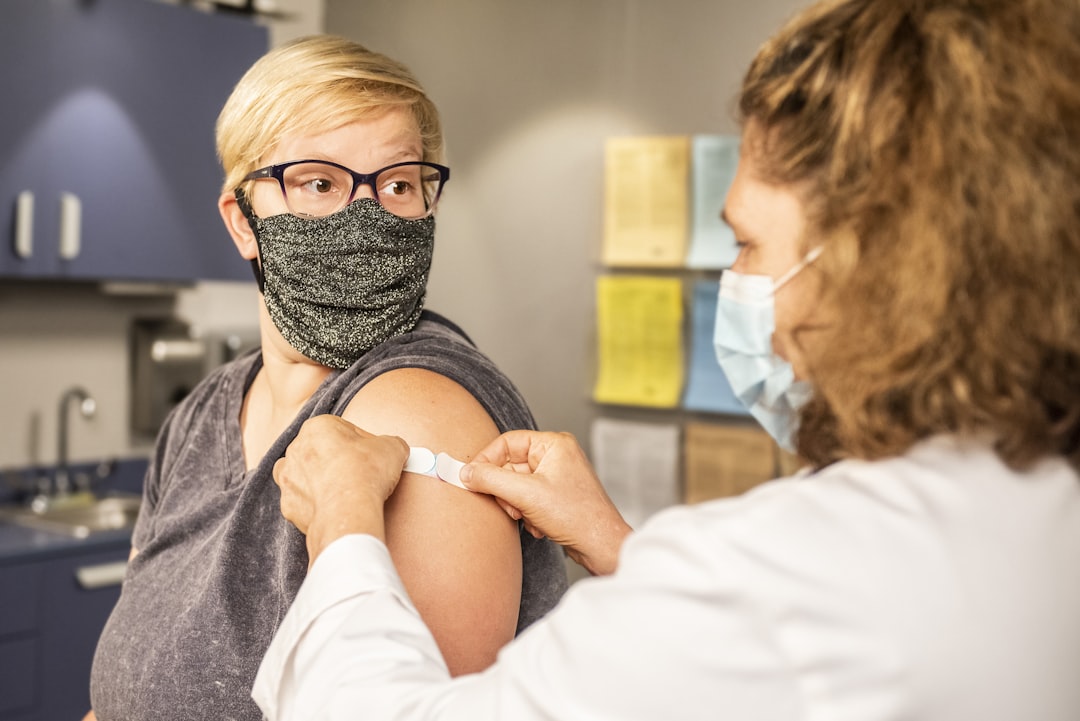
Strain-agnostic vaccines have the potential to revolutionize global health by offering protection against any strain of a virus. This innovative technology could make vaccine development faster and more efficient, as researchers no longer need to predict and chase specific virus strains. Early evidence suggests these vaccines are safe for use in babies and immunocompromised individuals, a significant breakthrough. As the World Health Organization works to prioritize vaccines through the Immunization Agenda 2030, strain-agnostic solutions could play a crucial role in preventing infectious diseases and responding to emerging variants.
Revolutionary Approach to Immunization - Scientists have developed a groundbreaking vaccine technology that can effectively target any strain of a virus, eliminating the need to predict and chase specific strains each year.
Unlocking Safety for Vulnerable Populations - This innovative strain-agnostic vaccine has been demonstrated to be safe for use even in babies and immunocompromised individuals, expanding the reach of immunization programs.
WHO's Commitment to Variant-Proof Vaccines - The World Health Organization is actively working to prioritize the development and implementation of these strain-agnostic vaccines as part of the Immunization Agenda
Effective Against Emerging Strains - Studies have shown that the strain-agnostic vaccine approach can effectively neutralize variants of concern, such as the N501Y strain, providing enhanced protection against re-infections.
A Versatile Vaccine Platform - The strain-agnostic vaccine technology is based on an RNA-based strategy, developed by scientists at UC Riverside, which allows for rapid and efficient vaccine development.
Global Collaboration for Variant-Updated Vaccines - A workshop organized by the International Coalition of Medicines Regulatory Authorities and the WHO has highlighted the importance of strain-agnostic vaccines and their potential to mitigate the risks posed by future variants.
What else is in this post?
- Vaccine Breakthrough A Strain-Agnostic Solution Revolutionizing Global Health - Strain-Agnostic Vaccines: Unlocking Global Health Potential
- Vaccine Breakthrough A Strain-Agnostic Solution Revolutionizing Global Health - mRNA Technology: A Breakthrough in Vaccine Adaptability
- Vaccine Breakthrough A Strain-Agnostic Solution Revolutionizing Global Health - Adjuvants and Delivery Systems: Enhancing Vaccine Efficacy
- Vaccine Breakthrough A Strain-Agnostic Solution Revolutionizing Global Health - Targeted Antigens: Precision Strikes Against Infectious Diseases
- Vaccine Breakthrough A Strain-Agnostic Solution Revolutionizing Global Health - Rapid Response Capabilities: Combating Outbreaks Swiftly
- Vaccine Breakthrough A Strain-Agnostic Solution Revolutionizing Global Health - Boosting Immunity: Extending Vaccine Protection through Boosters
Vaccine Breakthrough A Strain-Agnostic Solution Revolutionizing Global Health - mRNA Technology: A Breakthrough in Vaccine Adaptability
The mRNA technology represents a significant breakthrough in vaccine adaptability, revolutionizing global health. This strain-agnostic solution can protect against various viral strains, including COVID-19, without relying on a modified version of the virus. mRNA vaccines have already proven highly effective in saving lives during the pandemic and are being tested for other infectious agents, highlighting their immense potential.
The success of mRNA vaccines lies in their ability to overcome the challenges faced by traditional vaccine platforms. By utilizing messenger RNA to deliver genetic material directly into cells, mRNA vaccines can be produced and delivered at a significantly faster rate, while still generating a robust and long-lasting immune response. This adaptability has been crucial in responding to rapidly evolving viral variants.
mRNA technology is a revolutionary approach that enables the rapid development of effective vaccines against infectious diseases.
Unlike traditional vaccine platforms, mRNA vaccines do not require the use of weakened or inactivated viruses, which streamlines the manufacturing process and reduces the risk of contamination.
The strain-agnostic nature of mRNA vaccines is a game-changer in the fight against emerging and rapidly evolving viral threats.
This adaptability allows for quick adjustments to target different variants, making mRNA technology a versatile solution in the global health landscape.
mRNA vaccines have demonstrated remarkable effectiveness in protecting individuals from COVID-19, a testament to the potential of this innovative technology.
The ability to trigger a robust and long-lasting immune response is a key advantage over conventional vaccine approaches.
The development of mRNA vaccines has been propelled by groundbreaking scientific discoveries, recognized with the 2023 Nobel Prize in Physiology or Medicine.
This prestigious accolade underscores the transformative impact of this technology on global health.
As of 2024, there are 47 mRNA vaccines under development, with 23 of them currently in clinical trials.
This remarkable progress highlights the immense potential of mRNA technology to address a wide range of infectious diseases, including influenza, Ebola, and Zika virus.
The speed and adaptability of mRNA vaccines have been crucial in the fight against COVID-19, allowing for rapid response to emerging variants.
This agility is a significant advantage in a constantly evolving pandemic landscape.
While mRNA technology has revolutionized vaccine development, critical challenges remain in terms of large-scale manufacturing, distribution, and ensuring global accessibility.
Ongoing research and collaboration are essential to address these hurdles and unlock the full potential of this transformative approach.
Vaccine Breakthrough A Strain-Agnostic Solution Revolutionizing Global Health - Adjuvants and Delivery Systems: Enhancing Vaccine Efficacy
Enhancing Vaccine Efficacy through Innovative Adjuvants and Delivery Systems
Recent advancements in vaccine research have led to the development of advanced adjuvants and delivery systems, significantly improving the efficacy and accessibility of vaccines. Proven adjuvant platforms, such as aluminum and MF59, have been widely approved and help enhance the immune response. Emerging delivery systems, including nanocarriers, demonstrate the potential to further boost vaccine safety and effectiveness.
The search for a strain-agnostic solution to tackle vaccine breakthroughs is a revolutionary approach in global health. By targeting the less mutable components of viruses, these vaccines could provide broad protection against various strains, when combined with cutting-edge adjuvants and delivery methods. Companies like Gritstone Oncology and GeoVax are at the forefront of this innovative strategy, which holds the promise of enhancing pandemic preparedness and control.
Classical adjuvants like aluminum and MF59 have been widely approved for use in various vaccines, improving their efficacy by enhancing and modulating the immune response.
New delivery systems, such as nanocarriers, are being explored for their potential to enhance vaccine efficacy and safety, with some demonstrating high transfection efficiency.
A recombinant plant-based adjuvanted COVID-19 vaccine has shown high vaccine efficacy and safety, highlighting the potential of novel adjuvant platforms.
Researchers are exploring the use of lipid-based delivery systems in mRNA vaccines, which have demonstrated impressive results in terms of enhancing vaccine efficacy.
Adjuvants can help reduce the amount of antigen needed in a vaccine, decrease the number of doses required, and improve the duration of protection.
Delivery systems, such as liposomes, virosomes, and nanoparticles, ensure that the antigen is delivered to the appropriate site in the body for an effective immune response.
The development of strain-agnostic vaccines, which target the components of the virus that do not mutate as frequently, has the potential to provide broad protection against various strains of a virus, revolutionizing global health.
Companies like Gritstone Oncology and GeoVax are at the forefront of developing these strain-agnostic vaccines, which when combined with effective adjuvants and delivery systems, could significantly improve vaccine efficacy and provide long-lasting protection against various viral infections.
Vaccine Breakthrough A Strain-Agnostic Solution Revolutionizing Global Health - Targeted Antigens: Precision Strikes Against Infectious Diseases
This innovative approach, which includes mRNA vaccines and novel antigenic targets, adjuvants, and delivery systems, aims to provide more effective and rapid protection compared to traditional spike mRNA vaccines. Precision engineering of subunit vaccine particles and targeted antigens are emerging as promising strategies to enhance immune responses and combat diverse viral variants.
The newly developed SARS-CoV-2 Beta RBD DNA vaccine directly targets the antigen and offers protection against severe disease within just 10 days of vaccination, a significant improvement over traditional spike mRNA vaccines.
Advances in vaccine technology, including mRNA vaccines and novel antigenic targets, adjuvants, and delivery systems, are revolutionizing disease prevention, offering more effective and targeted solutions.
Protein subunit vaccines, especially in particulate format, have shown improved vaccine efficacy by inducing strong immune responses, making them effective in preventing infectious diseases.
Precision engineering of subunit vaccine particles for prevention is a promising approach, and targeted antigens have been shown to be retained on follicular dendritic cells (FDCs) longer, making them potent antigen-presenting cells for vaccine design.
Researchers have developed mRNA-based T-cell-inducing antigens that target multiple conserved regions of SARS-CoV-2, enriching human HLA-I epitopes, which enhances the breadth of the T-cell response and potentially offers protection against diverse viral variants.
mRNA-based vaccines enable precise targeting of antigen-presenting cells, such as dendritic cells, enhancing the effectiveness of the immune response.
Lipid nanoparticle formulations have proven effective in delivering mRNA antigens directly to antigen-presenting cells, minimizing the risk of degradation and immune activation outside target cells, thus enhancing the immunogenicity of the vaccine.
This targeted approach using lipid nanoparticles not only enhances the immunogenicity of the vaccine but also reduces the potential for side effects.
The strain-agnostic nature of this new vaccine approach means that there is no more need to chase individual strains, as seen with the SARS-CoV-2 Beta RBD DNA vaccine, which offers protection against severe disease regardless of the viral variant.
Vaccine Breakthrough A Strain-Agnostic Solution Revolutionizing Global Health - Rapid Response Capabilities: Combating Outbreaks Swiftly
Revolutionizing Global Health with Rapid Response Capabilities
The CDC's Global Rapid Response Team (GRRT) has emerged as a vital resource, mobilizing quickly to combat public health emergencies worldwide. With over 99,819 person-days deployed in 2,447 missions since 2015, the GRRT's swift interventions have helped contain outbreaks and save lives. Meanwhile, advancements in vaccine development have transformed disease prevention, with initiatives like the Coalition for Epidemic Preparedness Innovations (CEPI) leading the charge for rapid-response capabilities.
As the travel industry looks to a post-pandemic future, the need for robust global health infrastructure has never been more apparent. Mighty Travels PREMIUM remains committed to keeping our readers informed on the latest innovations and policy reforms shaping the future of international travel and public health security.
The CDC's Global Rapid Response Team (GRRT) has spent over 99,819 person-days deployed in more than 2,447 total mobilizations since 2015, responding to outbreaks of diseases like cholera and COVID-19 around the world.
Advances in vaccine development have revolutionized disease prevention, with rapid response vaccines for epidemic outbreaks being developed to combat emerging threats.
The COVID-19 pandemic has accelerated global collaboration and action, with international initiatives seeking to strengthen and reform the global architecture for pandemic preparedness and response.
Breakthrough cases of COVID-19 have been documented, but advances in vaccines have safeguarded public health and prevented infectious diseases.
The GRRT's expansion to the US response has enabled it to swiftly address outbreaks within the country, further enhancing global health protection.
The Coalition for Epidemic Preparedness Innovations (CEPI) supports preparedness activities for a rapid research response in areas essential for vaccine development.
Public health responses must invest in fast-acting and scalable systems in communities, requiring a frontline public health workforce capable of rapid response to outbreaks.
The Access to COVID-19 Tools (ACT) Accelerator has been proposed as a model for a pre-negotiated system to enable rapid response in future outbreaks, in conjunction with accountability mechanisms.
Sustaining robust vaccine manufacturing capacity built due to COVID-19 will keep it ready for rapid response in the future.
The CDC's Center for Global Health responds to outbreaks and works on adapting interventions for various diseases, including those preventable by vaccines.
Vaccine Breakthrough A Strain-Agnostic Solution Revolutionizing Global Health - Boosting Immunity: Extending Vaccine Protection through Boosters
As of April 24th, 2024, new research suggests that booster shots play a crucial role in extending protection against COVID-19. While primary vaccination offers initial protection, booster doses enhance the immune system's response, providing broader coverage against diverse variants. Interestingly, both breakthrough infections and booster shots can significantly boost the immune response, offering comparable enhancements in protection. Healthcare organizations and pharmaceutical companies have released data supporting the use of boosters to maintain long-term immunity and address potential breakthrough infections.
Booster shots can significantly enhance the quantity and quality of antibodies produced by the immune system, providing broader protection against diverse COVID-19 variants.
Studies have found that both breakthrough infections and booster doses can substantially boost the immune response, offering comparable enhancement in protection against the virus.
Data indicates that booster doses are highly effective in preventing severe disease and hospitalization, with a remarkable increase in protection reported in a large U.S.
study.
Healthcare organizations, such as the National Institutes of Health, and pharmaceutical companies, like Johnson & Johnson and Moderna, have released data supporting the use of boosters to maintain robust immune protection.
Booster shots can help address potential breakthrough infections, as protection against infection and transmission with variants like Omicron is only modest with the primary vaccine series.
While the initial vaccines provide good protection, their efficacy against the Delta variant is waning, highlighting the importance of booster doses.
Johnson & Johnson and Moderna have announced new data demonstrating that their boosters can improve and extend protection for those who received their primary vaccines.
The Pfizer-BioNTech vaccine booster has already been authorized for emergency use by the FDA for individuals aged 12 and older.
Researchers have observed that a breakthrough infection or a booster dose can both enhance the immune response against the virus, but getting boosted can result in "super immunity."
Booster shots targeting diverse variants, such as Omicron, provide broader protection and ensure sustained immunity against COVID-
The recommended timing for booster doses varies, with studies suggesting that protection may start waning around four to five months after the primary vaccine series.
